Review Tiers for Medical Marijuana Business License Applications
Tier 1: Preliminary Land Use requirements, Sales and Use Tax License and Background Checks (Licensing)*
Tier 2: Building Permit Review-Completion of Construction, Planning & Development Services (P&DS)*
Tier 3: Operating and Security Plan Review (P&DS)*
Tier 4: Physical Inspection of Premises, Compliance with Approved Plans (Licensing)*
Tier 5: Finalization of Operating Documents, Issuance of License (Licensing)* ( )* indicates the departments coordinating the tier process, however, approval may be required from several departments for each Tier
Tier 2 and 3 Requirements for Medical Marijuana Businesses
- An application for a building permit for a medical marijuana business will not be accepted without confirmation of passage of the First Tier Review by the city licensing office.
- The information contained in these Guidelines is in addition to all other applicable requirements for construction within the City.
- Separate from the standard drawings and materials required for building permit application, “Security” and “Operations” ww plans (with related narratives) are required and must include the following information, in the specific following format:
- Security Plan:
- 11” x 17” sheet size
- A dimensioned floor plan in which the medical marijuana business is to be located, drawn to accommodate the sheet size;
- The principal uses of the floor area labeled on the floor plan, including, but not limited to, the areas where non-patients will be permitted, private consulting areas, storage areas, retail areas, and areas where medical marijuana will be processed or distributed;
- The locations of all proposed exterior lighting and light fixture information;
- Location of cameras, motion detectors, security system computer;
- 7-day tape storage location;
- Lighting control information;
- Entrances and exits; and
- Locations of safes.
- Operation Plan (with attached narrative):
- 11” x 17” sheet size
- A dimensioned floor plan in which the medical marijuana business is to be located drawn to accommodate the sheet size;
- The principal uses of the floor area labeled on the floor plan, including, but not limited to, the areas where non-patients will be permitted, private consulting areas, storage areas, retail areas, and areas where medical marijuana will be processed or distributed;
- Entrances and exits; and
- A narrative that addresses the following:
- A description of the products and services to be provided by the business.
- A plan for ventilation of the medical marijuana business that describes the ventilation systems that will be used to prevent any odor of medical marijuana off the premises of the business. For cultivation facilities, such plan shall also include all ventilation systems used to control the environment for the plants and describe how such systems operate with the systems preventing any odor from leaving the premises.
- A description of all toxic, flammable, or other materials regulated by a federal, state, or local government with authority over the business that will be used or kept at the medical marijuana business, the location of such materials and how such materials will be used and stored.
- Security Plan:
Zoning Guidelines
Zoning approval is required for any building permit needed for work conducted to create a medical marijuana businesses as required in by B.R.C. 1981, 6-14-3 (b). Be advised that work associated with a proposed medical marijuana business may trigger additional improvements or may need to meet additional standards, including but not limited to:
- Site or building improvements may trigger the obligation to comply with landscape requirements
- Proposed exterior lighting will require the submittal of a compliant lighting plan
- All proposed mechanical equipment must be screened from view
Building Guidelines
A building permit is required for work conducted to create a medical marijuana business as required by B.R.C. 1981, 6-14-3 (b). The building permit application must meet the general building permit submittal requirements. The plans must be prepared by a Colorado Design Professional and must address specific medical marijuana related requirements including the following:
- Cultivation facilities must meet International Building Code (IBC) Chapter 3 requirements based on a Use and Occupancy Classification of Factory Industrial, F-1, Moderate-hazard Occupancy (IBC 306.2).
- Centers and dispensaries must meet IBC Chapter 3 requirements based on a Use and Occupancy Classification of a Mercantile Occupancy, M, or a Business Occupancy, B depending on the amount and level of treatment services provided (IBC 309.1).
- Demonstrate that the proposed establishment of the medical marijuana business will not compromise allowable area compliance of the building based on the applicable Type of Construction categorized in IBC Chapter 6.
- Applicable Means of Egress requirements based on IBC Chapter 10.
- Applicable Accessibility requirements based on IBC Chapter 11.
- Applicable Accessibility improvement requirements as specified by IBC sec. 3409.7
- Applicable fire suppression system requirements based on IBC Section 903 and local amendments. A change in the occupancy of the space or an expansion of square footage could require a fire suppression system requirement for the proposed space.
- Applicable flame spread ratings for Interior Finishes based on IBC Chapter 8 and F-1 Occupancy Group.
- Floor plans must be drawn to an easily readable scale. Relevant dimensions must be provided. Identify the use of all rooms. Specify hall and stair widths. Show the locations and types of plumbing fixtures as well as existing and proposed plumbing rough-ins.
- Stocking of medical marijuana is not permitted until a certificate of occupancy or letter of completion is issued and authorization is granted by the City Licensing division after the inspection of Tier 4 required for the medical marijuana business license.
Mechanical Guidelines
A ventilation system will be required to filter the odor from a business so that it cannot be detected at the exterior of the business or at any adjoining property as detailed in B.R.C. 1981, 6-14-8 (h). The ventilation system for a medical marijuana business requires, at a minimum:
- Exhaust systems designed and constructed to capture sources of contaminants to prevent spreading of contaminants or odors to other occupied parts of the building reference “Contaminant sources,” International Mechanical Code (IMC) 401.6.
- Building elements separating MMB from other occupied portions of the building must be air sealed to prevent odor migration into adjacent spaces.
- Cultivation facilities must have a ventilation rate of 60 cfm/person.
- Centers and dispensaries must have an outside ventilation rate of 15 cfm/person.
- Exhaust from the space must be filtered with a listed and labeled filter designed to remove odors from the exhaust stream.
- The inlet for the ventilation system must be located in the area(s) of highest contaminant concentration.
- Cultivation facility exhaust outlets must be 10 feet from property lines, operable openings into a building and from mechanical air intakes IMC 502.1.2. (1).
- Center facility exhaust outlets must be 3 feet from property lines, operable openings into a building and from mechanical air intakes IMC 502.1.2. (3).
- Additional requirements for cultivation facilities:
- Carbon dioxide generation systems must be listed and labeled, properly installed and functioning with a concentration level of no more than 1500 ppm.
- Light fixture exhaust duct work must be installed according to IMC requirements.
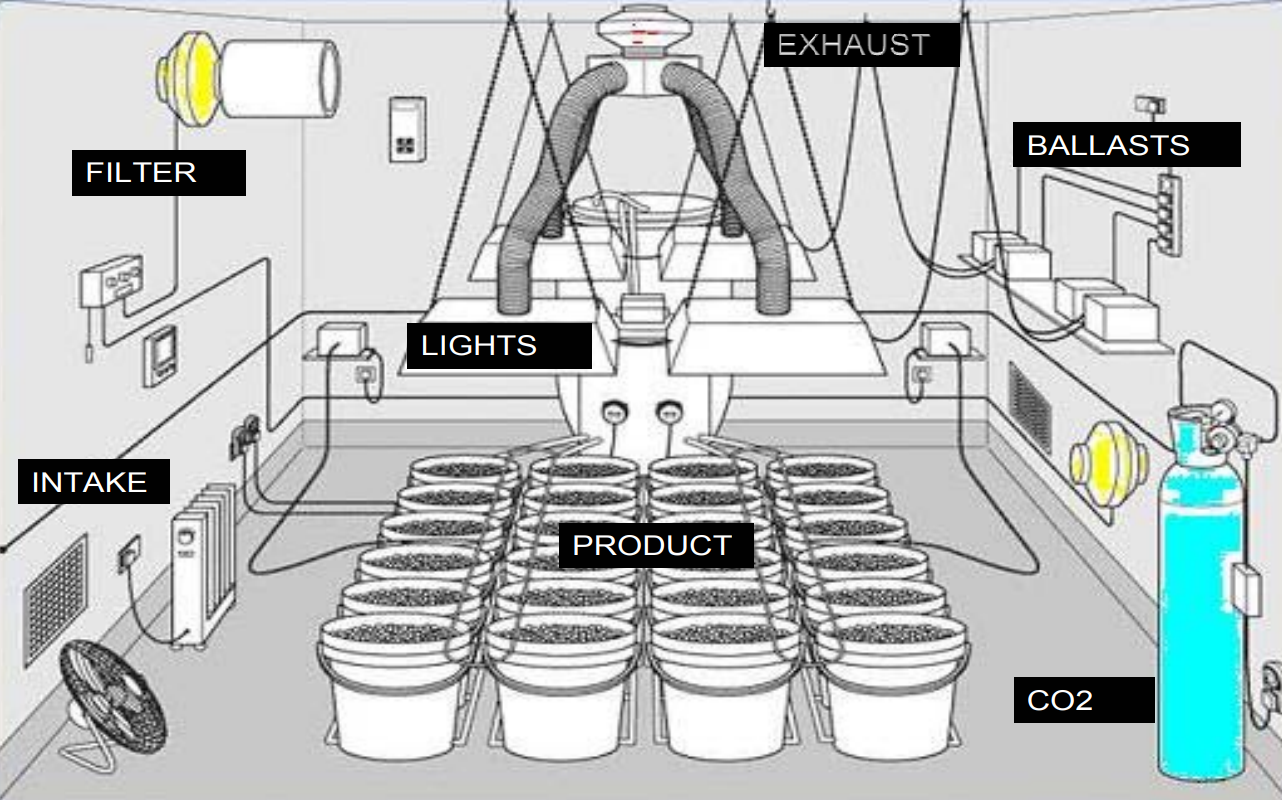
A schematic diagram illustrating the ventilation system design for a medical marijuana cultivation facility. The layout features various interconnected components, including labeled sections for air intake, filtration units, ductwork, fans, and exhaust outlets. Arrows indicate the direction of airflow through the system, which is designed to regulate temperature, humidity, and overall climate within the growing spaces. Key mechanical components such as cooling and heating units, air diffusers, and control elements are marked, demonstrating how conditioned air is distributed and exhausted to maintain an optimal environment for plant growth.
Energy Efficiency Guidelines
Every medical marijuana business shall:
- Meet the applicable energy efficiency requirements of the International Energy Conservation Code (IECC) and applicable local amendments (must be 30% better than the requirements detailed in the IECC), B.R.C. 1981, 10-7-2 (h). An approved “Energy Recovery Ventilation System,” IMC 514, could be utilized to fulfill these energy efficiency requirements.
Every cultivation facility shall:
- Directly offset 100% of its electricity consumption through the purchase of renewable energy in the form of Colorado Wind Source, a verified subscription in a community solar garden, or renewable energy generated on-site, or an equivalent that is subject to approval by the city, B.R.C. 1981, 6-14-8 (i).
Electrical Guidelines
All electrical systems and permitting are required to be done by licensed electricians and contractors, “Electrical Contractor Registration,” B.R.C. 4-8-1. In addition:
- A single line diagram of the existing and proposed electrical system, including the main electrical service National Electric Code (NEC) 215.5, shall be provided to the city. Electrical services which are 400 amps or greater must be provided by a Colorado Design Professional.
- All electrical equipment is to be listed and labeled by an approved testing agency.
- Flexible cords (extension cords) are not permitted to substitute for fixed wiring and can not be routed through or concealed in walls, structural ceilings, suspended ceilings, dropped ceilings or floors, attached to building surfaces, or be within 6’-8” of a means of egress.
- Approved wiring methods utilized in grow facilities in accordance with “Wiring Methods and Materials."
- NM cable (romex) is not allowed for use in damp locations like grow facilities.
- All electrical equipment and building components shall be completed, inspected and approved prior to stocking of any medical marijuana.
Medical Infused Product Guidelines
An industrial hygienist and/or State licensed design professional shall provide detailed plans and specifications on the process for extracting canibinoids from marijuana plant products with flammable solvents, gasses and solids per IBC 307, IMC 510 and NEC chapter 5. Additionally, sanitation shall be maintained through design and implementation of the guidelines detailed below.
- Concentrations of flammable liquids and gasses in excess of 25 % of the lower flammability level of the products will require a system designed in compliance with IMC section 510 to mitigate the potential for explosion or fire (see also IBC section 307 and IFC chapters 34 – 37)
- Concentrations of grease, smoke, heat, steam or products of combustion created when medical marijuana products are processed into foods, beverages, salves, inhalants and tinctures are to be contained as detailed in the IMC sections 506 and 507 (Type I and Type II hoods)
- Sanitation requirements for facilities used for processing medical marijuana into foods, beverages, salves, inhalants and tinctures shall meet the following guidelines that are to be detailed on the drawings submitted for review
- Location of hand wash sinks;
- How dishes will be washed. IPC section 802.1 requires all food handling and health care related fixtures, devices, and equipment to discharge through indirect waste lines into a floor sink;
- Contact surfaces shall be smooth, free of breaks, open seams, cracks, chips, pits and similar imperfections, free from sharp internal angles, corners, crevices, finishes to have smooth welds and joints;
- Equipment containing bearings and gears shall be designed, constructed and maintained to ensure that it meets food and health requirements (washing machines are not listed for food or health related preparations);
- All rooms shall have sufficient ventilation to keep them free from excessive heat, steam, condensation, vapors, odors, smoke and fumes per IMC chapters 4 and 5;
- Table or counter mounted equipment shall be installed to facilitate the cleaning of the equipment and adjacent areas by being sealed to the surface or elevated by at least four inches;
- Three compartment sinks are required for washing, rinsing, and sanitizing equipment and utensils;
- Hand sinks must be conveniently located for employees;
- Hand sinks shall only be used for hand washing (maximum water temperature of 110 degrees is to be maintained through an appropriate mixing valve);
- Sinks used for food or medicine preparation or for washing equipment shall not be used for hand washing;
- At least one utility or mop sink must be provided;
- Garbage and refuse shall be stored in a manner to be inaccessible to insects and rodents;
- Floors shall be smooth, durable, nonabsorbent, light colored and maintained in good repair;
- The junction between the floor and wall shall be closed and sealed; and
- Walls and ceilings must be smooth and easily cleanable
Infused product extraction and hazard containment equipment must be listed, labeled and installed per NEC 110.3 and IMC 301.4.